FUNDAMENTAL RESEARCH
The main objective of fundamental research is to produce knowledge and understanding in relation to natural phenomena. In life and health sciences the particular focus is on deciphering the mechanisms of life, and understanding not just the functioning of the human body, but also that of organisms and any other entity with which it interacts.
This exploratory research relies on the curiosity and creativity of researchers. Taking their departure from available knowledge and their own observations, scientists develop questions and hypotheses concerning the mysteries that still remain. To test these hypotheses, they develop experiments that will lead them to generate new data.The results of such research are hard to predict, and researchers will not always find an answer to their original question. But they sometimes discover things that help answer other questions, or may even come across totally unexpected new phenomena! In all cases, fundamental research contributes to the growth of knowledge.
When seeking innovation, it is always possible to improve upon what already exists. But the most significant progress is most often based on discoveries generated by fundamental research. Without studies on basic immunological mechanisms, immunotherapy would never have been possible. And yet this therapeutic approach has been a game changer in the management of certain cancers. Another example is provided by MRI: a medical imaging technique now in frequent use that was developed as a result of fundamental and theoretical work on nuclear magnetic resonance.
CLINICAL RESEARCH
Clinical research includes all scientific studies conducted on human beings, with a view to developing biological or medical knowledge. These research studies are essential to better understanding and/or better treating diseases, as well as to identifying potential risk factors. Eventually, they help to improve our health.
CLINICAL TRIAL
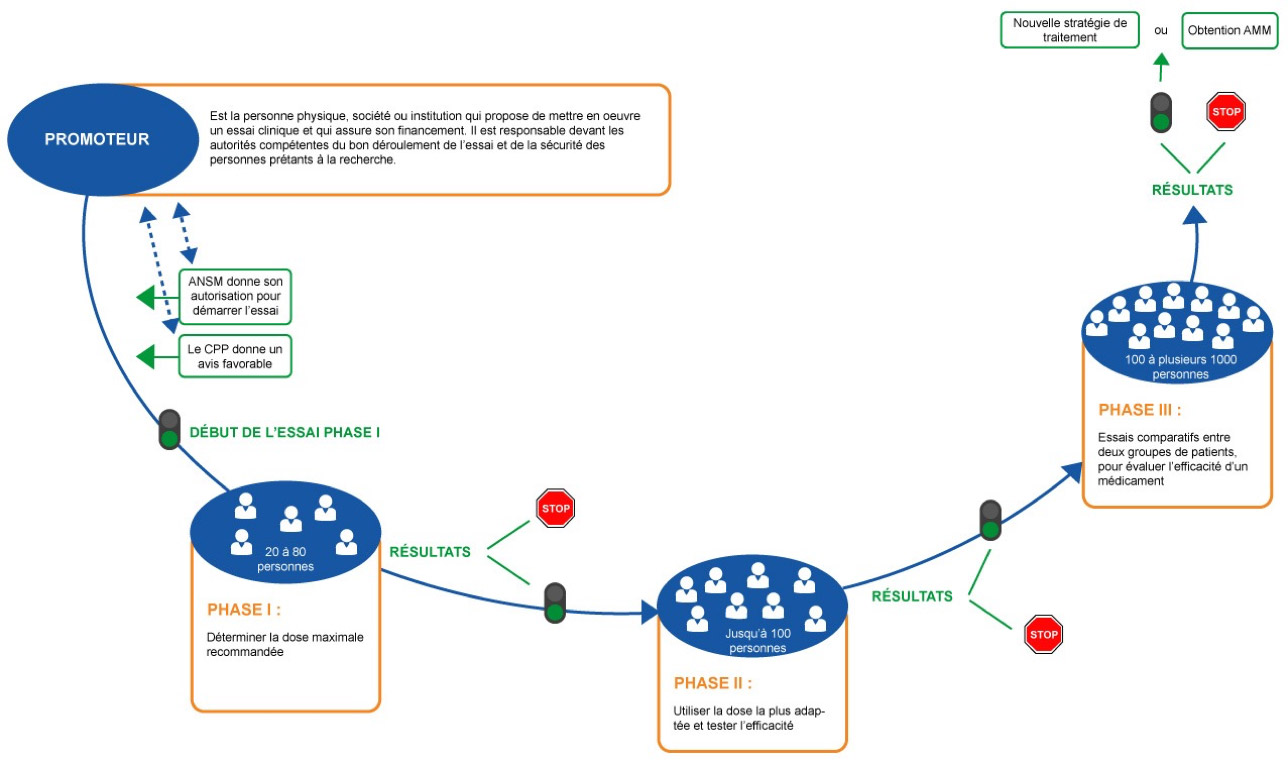
A clinical trial is research done on humans to assess the effectiveness and tolerance of a diagnostic method, a treatment or a medical device.
The trial may be done on diseased volunteers or on healthy volunteers.
To start, the trial must have received a favourable opinion from the Comité de Protection des Personnes (C.P.P. (Committee for the Protection of People) and an authorisation from the Agence Nationale de Sécurité du Médicament (ANSM or the French National Agency of Safety of Medication and Health Products).
Trial participants have the following rights:
-
the right to clear and precise information throughout the duration of the trial;
-
the right to refuse to participate, without impacting their medical treatment;
-
the right to confidentiality, i.e. the data collected will only be accessible by authorised people;
-
the right to anonymity, i.e. the patient's identity is not known to the promoter;
-
the right to withdraw from the trial at any time without having to justify this decision and without consequence on their medical treatment;
-
the right to access, change and delete data collected, in compliance with the Information Technology, Data Files and Civil Liberties Act;
-
the right to be compensated in case of damages resulting from the trial;
-
the right to the communication of general test results, if they so wish.
Three different types of clinical research on humans are defined in a law called the "Jardé Act":
-
Interventional research (category 1)
This involves an intervention not devoid of risk for participants and not justified by their normal treatment. This can be research on medications, medical tools, gene or cell therapies and/or surgical techniques.
-
Interventional research with minimal risk and constraints (category 2)
This concerns studies involving non-invasive interventions or actions, whose list is set by a governmental order.
-
Non-interventional research (category 3)
Despite the name, this research includes acts or procedures listed by government order but without risk and not changing the treatment of participants, and all acts performed and products used are done so normally.
For each clinical trial, an investigator is designated to manage and monitor the proper performance of the trial.
For clinical trials of medications, the investigator is a physician with appropriate experience. The latter works in collaboration with a multi-disciplinary team (clinical trial technicians, nurses, physicians, radiologists, pharmacists, biologists, etc.) also trained in clinical research.
CLINICAL TRIAL PHASES
The clinical evaluation relies on four phases:
Phase I: first test in humans on a small population generally staying for a few days in a hospital environment. It helps establish the tolerance of the organism to the treatment in order to define a recommended dose;
Phase II: determines the effectiveness of the treatment and its potential undesirable effects;
Phase III: final phase before market release, which may last several years, this is a "comparative" phase helping to assess the effectiveness of the treatment on a relatively large cohort of patients. Volunteers are most often divided into two groups in order to compare the effectiveness of the candidate medication to a benchmark treatment (if there is one) or a placebo.
Phase IV: If the treatment proves to be safe and effective, an Autorisation de Mise sur le Marché (AMM or Market Release Authorisation) must then be obtained in order for it to be commercialised.
After their market release, medications continue to be the subject of a strict long term follow-up, called post-AMM, in order to identify any serious and/or unexpected side effects due to their administration. This is called pharmacovigilance.
CLINICAL RESEARCH IN FIMATHO NETWORK
In order to maintain their level of expertise but also to improve patient care and knowledge about rare abdomino-thoracic diseases, the centres of reference in the FIMATHO network are carrying out clinical research projects.
Click on the links below to find out about the research projects of the various centres of reference in the FIMATHO network:
Sources : INSERM – Notre Recherche Clinique – ANSM – Alliance Maladies Rares – Ligue-cancer


